Paxlovid
Studies from the drugmaker showed that in unvaccinated people at risk of serious COVID. 1For information on medical conditions and factors associated with increased risk for progression to severe COVID-19.
Here S The Latest Good And Bad News About Covid 19 Drugs
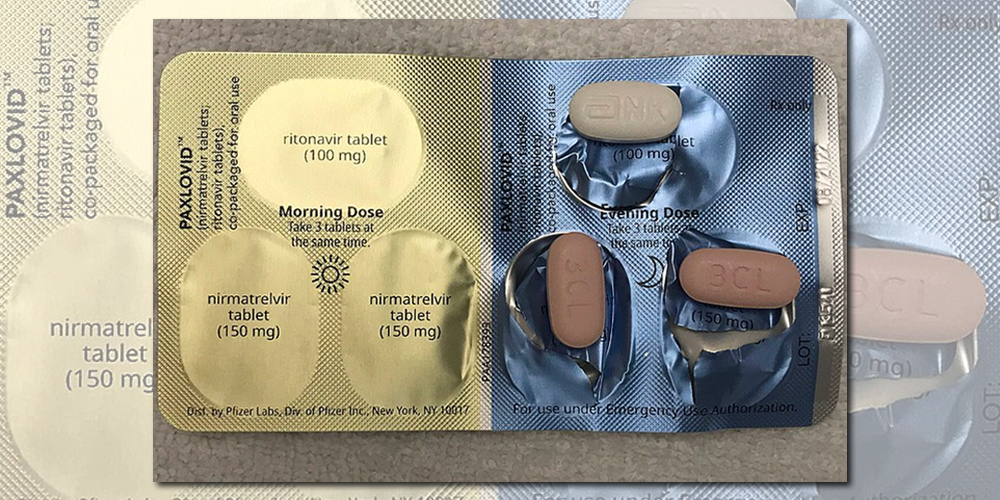
Paxlovid is for individuals age 12 and older who have tested positive for COVID-19 are at increased risk of severe disease and have developed COVID-19 symptoms in the last 5 days even mild ones such as runny nose or cough.

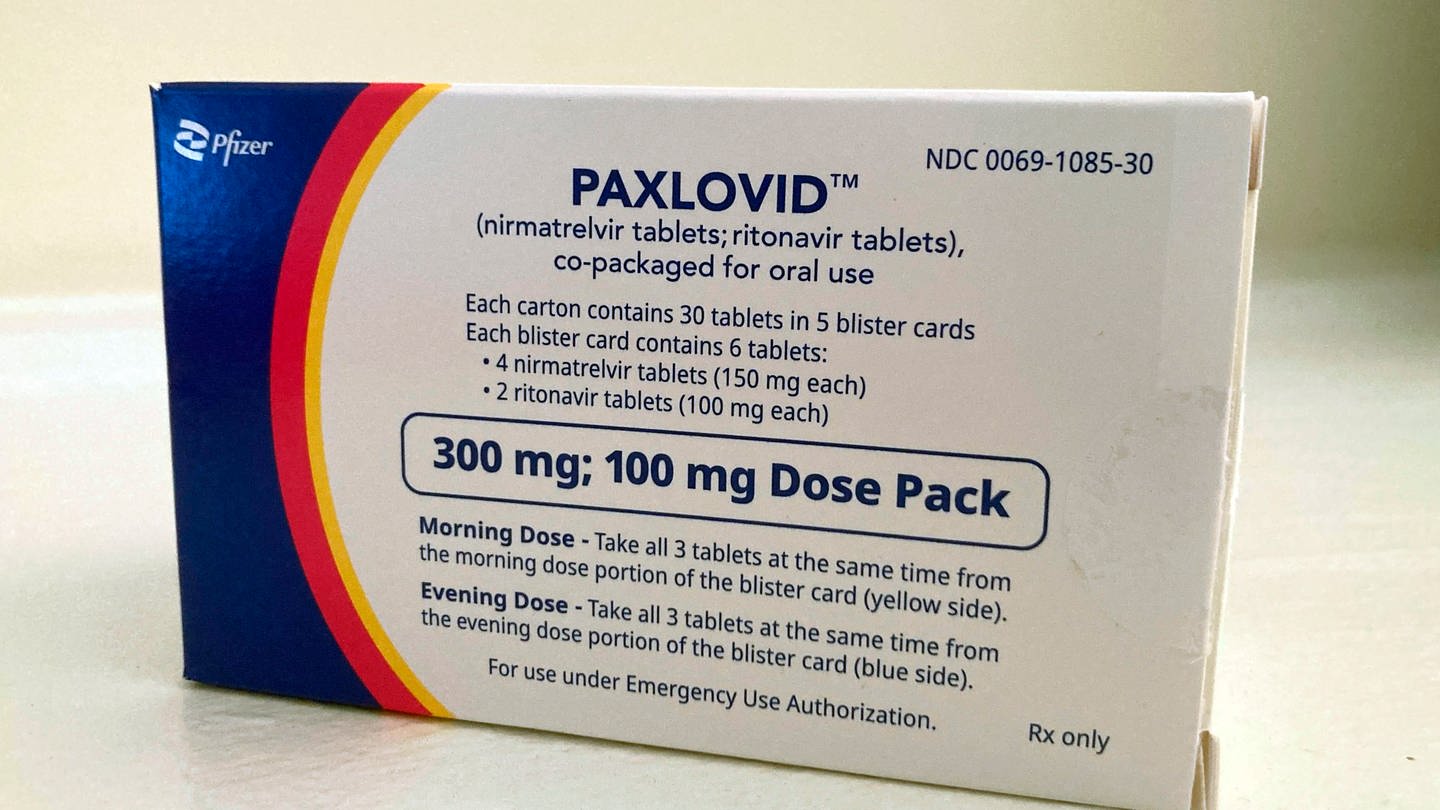
. Paxlovid Find out how Paxlovid treats coronavirus COVID-19 and how to take it. It found taking Paxlovid orally within the first five days of infection was tied to a 25 relative decrease in developing 10 of the 12 most common long COVID symptoms including shortness of. Paxlovid is also contraindicated with drugs that conversely strongly induce those same enzymes leading to the faster breakdown of nirmatrelvir or ritonavir as reduced concentrations of.
Call 1-800-232-0233 TTY 1-888-720-7489 to get help in English Spanish and more than 150 other languages8 AM to midnight ET 7 days a week. Paxlovid eligibility refer to FDAs Fact Sheet for Healthcare Providers. In addition the three oral drugs did not increase the occurrence of adverse events thus.
Improving patient care through guidelines evidence summaries and decision aids that we can all trust use and share. Ritonavir tablets Adverse Event Report. Paxlovid was granted emergency use authorization for the treatment of mild to moderate COVID-19 based on the interim analysis of EPIC-HR trial.
We have produced various materials in PDF format to aid the use of experimental agents in the treatment of COVID-19. Since the trigger of long COVID is acute infection with SARS-CoV-2 it makes intuitive sense. Ontario is considering allowing pharmacists to prescribe the COVID-19 treatment drug Paxlovid in order to expand access the provinces top doctor says.
Due to the potential for severe drug-drug interactions with the ritonavir component of Paxlovid it is strongly suggested that healthcare providers not experienced in prescribing this drug refer to. PAXLOVID is not an appropriate therapeutic option based on the authorized Fact Sheet for Healthcare Providers or due to potential drug interactions for which recommended monitoring would not be feasible. The new study says that Paxlovid may help stave off some of those issues.
Whether you are a patient caregiver or healthcare professional it is important to report adverse events for Pfizer products. In late April a study found that Paxlovid wasnt successful as a preventive measure for people who had been exposed to the virus. Food and Drug Administration revised the Emergency Use Authorization EUA for Paxlovid nirmatrelvir and ritonavir to authorize state-licensed pharmacists to prescribe.
National Center for Biotechnology Information. Paxlovid effectiveness needs to be assessed in a noncontrolled setting. In this study we used population-based real world data to evaluate the effectiveness of Paxlovid.
What Prescribers and Pharmacists Need to Know. These resources are freely available and the Liverpool Drug Interactions Group would like to encourage the dissemination with an appropriate acknowledgement of this drug-drug interaction information for non-commercial use. Also known as PAXLOVID nirmatrelvir tablets.
Ontario COVID-19 Drugs and Biologics Clinical Practice Guidelines Working Group on behalf of the Ontario COVID-19 Science Advisory Table. The study shows the challenge of demonstrating a benefit of antiviral therapy in previously vaccinated or otherwise low risk individuals said Paul Sax clinical director of the Division of Infectious. Past studies have shown that Paxlovid reduces the risks of hospitalization and death from COVID-19.
6 2022 Read the full story here. Were the NPR and PBS member station for Northern California. Paxlovid a five-day course of pills from Pfizer is at the top of the list of recommended treatments.
National Center for Biotechnology Information. KQED provides public radio television and independent reporting on issues that matter to the Bay Area. About Paxlovid Who can and cannot take it How and when to take it Side effects Pregnancy breastfeeding and fertility Taking Paxlovid with other medicines and herbal supplements.
One rising variant BA2752 is particularly concerning for its potential to thwart the last monoclonal antibody left in the COVID arsenal. Call the Centers for Disease Control and Prevention CDC COVID-19 hotline which can provide information on the Test to Treat initiative. This study showed that three novel oral antivirals molnupiravir fluvoxamine and Paxlovid are effective in reducing the mortality and hospitalization rates in patients with COVID-19.
Researchers found the decreased risk of long COVID associated with the medication exists regardless of whether it was a. Learn how FDA prepares for and responds to emergencies and how FDA plays a role in protecting the United States from chemical biological radiological nuclear and emerging infectious disease.

Covid 19 Arzneimittel Paxlovid Sup Sup Richtig Abrechnen Das Pta Magazin
Yxmdl4si L2hgm

Arzte Konnen Paxlovid Ab Freitag Verordnen
Covid Medikament Karl Lauterbachs Paxlovid Offensive Zdfheute

Bremer Pharmakologe Nennt Schnelle Paxlovid Zulassung Hanebuchen Buten Un Binnen

Tabletten Zur Behandlung Von Covid 19 Was Die Corona Medikamente Paxlovid Und Molnupiravir Versprechen Deutschlandfunk De

Paxlovid Und Co Expertenrat Fordert Besseren Einsatz Von Corona Medikamenten Der Spiegel

Intensivmediziner Fur Vermehrte Behandlung Von Coronapatienten Mit
Corona Medikament Kaum Verschrieben Dr Barbara Romer Paxlovid Is Kein Medikament Der Masse Swr1
Paxlovid Bei Covid 19 Cochrane Deutschland

Paxlovid Has Been Free So Far Next Year Sticker Shock Awaits

Paxlovid For Covid 19 Essential Info Everyday Health
Sndmjoirjdsp M

Evaluating Differences In Viral And Symptom Rebounds Between Paxlovid Treated And Untreated Covid 19 Patients
Racgp Paxlovid Goes On Pbs As Gps Urged To Avoid Private Prescribing

Paxlovid Warum Die Tablette Gegen Corona Kaum Genutzt Wird Zeit Online
Pfizer To Deliver Up To Six Million Courses Of Paxlovid To Global Fund